


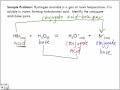















The conjugate base contains one less H than its corresponding acid and 1 more negative charge than its corresponding acid. So, the conjugate base for H2CO3 H 2 C O 3 HCO− 3 H C O 3 −. TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a (25 oC) HClO 4 ClO 4 – H 2 SO 4 HSO 4 – HCl Cl– HNO 3 NO 3 – H 3 O + H 2 O H 2 CrO 4 HCrO 4 – 1.8 x 10–1 H 2 C 2 O 4 (oxalic acid) HC 2 O 4 – 5.90 x 10–2 [H 2 SO 3] = SO 2 (aq) + H2 O HSO What is conjugate base of H2CO3? There is no such ion as H2CO3- However, the neutral molecule H2CO3 exists. Its conjugate base is the bicarbonate, or hydrogen carbonate ion: HCO3- The conjugate The conjugate base of H 2 CO 3 is HCO 3 - . To determine the conjugate base, remove a proton (H +) from the acid. The formula will have one less hydrogen... See full answer below. Answer. The conjugate base of bicarbonate, HCO 3- is carbonate, CO3 2-. HCO3- is a conjugate acid, H 2 CO 3. Log in or register to post comments. 3) Identify the acid, base, conjugate acid, and conjugate base of HSO4 + NH3 -----> SO4 + NH4 Acid: HSO4 Base: NH3 Conjugate acid: NH4 Conjugate base: SO4 Got the wrong answer? Click Here to review Click Here to go back to the QUIZ!! 4) Identify the acid, base, conjugate acid, and conjugate base of C2H3O2 + HCl -----> C2H4O2 + Cl Acid: HCL Base acid base base acid. here H2CO3 and HCO3- are conjugate acid-base pair as are H2O and OH-b) HCl + H 2 PO 4-<-----> Cl - + H 3 PO 4. HCl transfers a proton (H+ ion) to H 2 PO 4- and form Cl - and H 3 PO 4, now Cl - can accept a proton donated by H 3 PO 4 .so the above equation is; Identify the conjugate base in the reaction HCO3- + HPO4-2 <---> H2CO3 + PO4-3 H2CO3 Arrange the acids HOBr, HBrO3, and HBrO2 in order of increasing acid strength, Conjugated bases always have one proton less than its (conjugated) acids:So the conjugated base of carbonic acid ( H2CO3 ) is: hydrogen carbonate, formula HCO3-
[index] [2617] [8208] [7358] [6286] [6921] [4734] [6196] [7089] [4698] [8336]
Pyruvic acid is placed in water at physiological pH (7.3). Under these conditions, which species will dominate; the conjugate base or the conjugate acid? The... Conjugate acids and bases are usually introduced in organ... Skip navigation Sign in. ... How To Find The Conjugate Base - Duration: 1:51. The Organic Chemistry Tutor 62,415 views. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One... This chemistry video tutorial explains the concept of acids and bases using the arrhenius definition, bronsted - lowry and lewis acid base definition. It al... Brønsted - Lowry definition, strong and weak, conjugate acid-base pairs About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... +2 pts per boxconjugate base stabilization increases acid strength
Copyright © 2024 hot.toprealmoneygames.xyz